Objective: To estimate the effect of rivastigmine therapy on time to first antipsychotic drug prescription among patients with Alzheimer's disease (AD).
Design: We conducted a retrospective cohort study using patients identified from MarketScan® research databases during 1/1/1999-3/31/2002. These databases include longitudinal data from medical service and prescription drug claims from nearly 100 insurance companies in the U.S.
Materials and Methods: We included patients ≥65 years old if they were newly diagnosed with AD at 2 separate times or if they initiated rivastigmine during an 18 month index period. We excluded patients if they used any antipsychotics on or before initial AD diagnosis or first rivastigmine use. Rivastigmine users with <60 days of rivastigmine therapy were also excluded. We assigned patients either to a cohort of rivastigmine users or non-ChEI users. Differences in time to first antipsychotic use were tested using the log-rank test. A Cox proportional hazards model estimated rivastigmine's effect on time to first antipsychotic drug use, controlling for age, gender, and use of prior and concomitant central nervous system agents other than antipsychotics.
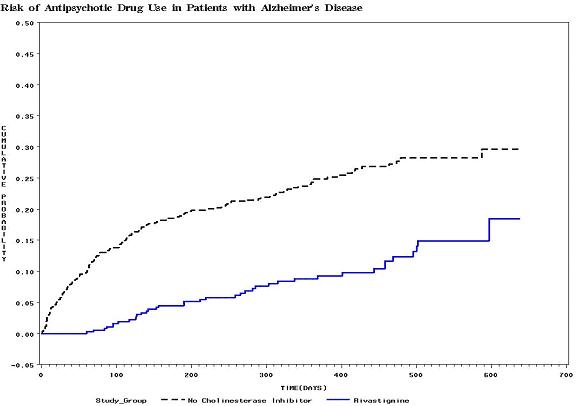
Results: The study identified 978 patients (rivastigmine cohort=370; non-ChEI cohort=608). By the end of the observation period (median time=395 days), 10.3% of the rivastigmine cohort had been prescribed an antipsychotic, compared with 24.7% of the non-ChEI cohort (p<0.0001). In the multivariable Cox regression model, the risk of being initiated on an antipsychotic drug was significantly lower for patients in the rivastigmine cohort. Rivastigmine users were 60% less likely to be prescribed antipsychotics than patients not on a ChEI (relative-risk (RR)=0.40; 95%CI: 0.28-0.58).
Conclusion: Patients treated with rivastigmine have a reduced risk of being initiated on an antipsychotic drug. This suggests that rivastigmine may delay the progression of behavioral symptoms of AD.
Back to PD Thursday Poster Sessions
Back to The Eleventh International Congress